What is a lithium-ion battery and how does it work?
A lithium-ion (Li-ion) battery is an advanced battery technology that uses lithium ions as a key component of its electrochemistry. During a discharge cycle, lithium atoms in t he anode are ionized and separated from their electrons. The lithium ions move from the anode and pass through the electrolyte until they reach the cathode, where they recombine with their electrons and electrically neutralize. The lithium ions are small enough to be able to move through a micro-permeable separator between the anode and cathode. In part because of lithium’s small size (third only to hydrogen and helium), Li-ion batteries are capable of having a very high voltage and charge storage per unit mass and unit volume.
he anode are ionized and separated from their electrons. The lithium ions move from the anode and pass through the electrolyte until they reach the cathode, where they recombine with their electrons and electrically neutralize. The lithium ions are small enough to be able to move through a micro-permeable separator between the anode and cathode. In part because of lithium’s small size (third only to hydrogen and helium), Li-ion batteries are capable of having a very high voltage and charge storage per unit mass and unit volume.
Li-ion batteries can use a number of different materials as electrodes. The most common combination is that of lithium cobalt oxide (cathode) and graphite (anode), which is most commonly found in portable electronic devices such as cellphones and laptops. Other cathode materials include lithium manganese oxide (used in hybrid electric and electric automobiles) and lithium iron phosphate. Li-ion batteries typically use ether (a class of organic compounds) as an electrolyte.
What are some advantages of Li-ion batteries?
Compared to the other high-quality rechargeable battery technologies (nickel-cadmium or nickel-metal-hydride), Li-ion batteries have a number of advantages. They have one of the highest energy densities of any battery technology today (100-265 Wh/kg or 250-670 Wh/L). In addition, Li-ion battery cells can deliver up to 3.6 Volts, 3 times higher than technologies such as Ni-Cd or Ni-MH. This means that they can deliver large amounts of current for high-power applications, which has Li-ion batteries are also comparatively low maintenance, and do not require scheduled cycling to maintain their battery life. Li-ion batteries have no memory effect, a detrimental process where repeated partial discharge/charge cycles can cause a battery to ‘remember’ a lower capacity. This is an advantage over both Ni-Cd and Ni-MH, which display this effect. Li-ion batteries also have low self-discharge rate of around 1.5-2% per month. They do not contain toxic cadmium, which makes them easier to dispose of than Ni-Cd batteries.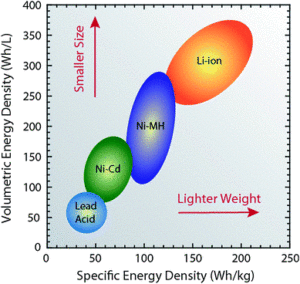 Due to these advantages, Li-ion batteries have displaced Ni-Cd batteries as the market leader in portable electronic devices (such as smartphones and laptops). Li-ion batteries are also used to power electrical systems for some aerospace applications, notable in the new and more environmentally friendly Boeing 787, where weight is a significant cost factor. From a clean energy perspective, much of the promise of Li-ion technology comes from their potential applications in battery-powered cars. Currently, the bestselling electric cars, the Nissan Leaf and the Tesla Model S, both use Li-ion batteries as their primary fuel source.
Due to these advantages, Li-ion batteries have displaced Ni-Cd batteries as the market leader in portable electronic devices (such as smartphones and laptops). Li-ion batteries are also used to power electrical systems for some aerospace applications, notable in the new and more environmentally friendly Boeing 787, where weight is a significant cost factor. From a clean energy perspective, much of the promise of Li-ion technology comes from their potential applications in battery-powered cars. Currently, the bestselling electric cars, the Nissan Leaf and the Tesla Model S, both use Li-ion batteries as their primary fuel source.
What are some disadvantages of Li-ion batteries?
Despite their technological promise, Li-ion batteries still have a number of shortcomings, particularly with regards to safety. Li-ion batteries have a tendency to overheat, and can be damaged at high voltages. In some cases this can lead to thermal runaway and combustion. This has caused significant problems, notably the grounding of the Boeing 787 fleet after onboard battery fires were reported. Because of the risks associated with these batteries, a number of shipping companies refuse to perform bulk shipments of batteries by plane. Li-ion batteries require safety mechanisms to limit voltage and internal pressures, which can increase weight and limit performance in some cases. Li-ion batteries are also subject to aging, meaning that they can lose capacity and frequently fail after a number of years. Another factor limiting their widespread adoption is their cost, which is around 40% higher than Ni-Cd. Addressing these issues is a key component for current research into the technology. Finally, despite the high energy density of Li-ion compared to other kinds of batteries, they are still around a hundred times less energy dense than gasoline (which contains 12,700 Wh/kg by mass or 8760 Wh/L by volume).
CEI Contributions
Research Highlights
One way that CEI has worked to accomplish this is through direct imaging, specifically using x-ray spectroscopy. Recently, Professor Jerry Seidler’s lab has developed a method to perform X-ray absorption near edge structure (XANES) spectroscopy on the benchtop. The technique can allow relatively detailed measurements of certain characteristics of a battery’s internal state, without having to open it and thus disrupt the system. Previously, XANES could only be accomplished with an extremely high radiative flux, from instruments such as a synchrotron. These are extremely large, expensive facilities, costing up to $1 billion, and are in such high demand among scientists that months-long waiting lists are the norm. Taking advantage of new, cutting-edge optical technologies, Seidler’s lab has been able to fabricate a small, $25,000 instrument that can mimic the measurements taken at a synchrotron. With this new instrument, scientists can have results in hours without significant waiting time, greatly increasing the speed of development for contingent technologies.
Another aspect of CEI battery research involves creating physical, mathematical and computational models for the battery’s internal states. This can help both optimize battery performance and charge/discharge cycles, as well as help predict and prevent dangerous battery failures. Professor Venkat Subramanian, who runs the Modeling, Analysis and Process-control Laboratory for Electrochemical Systems (M.A.P.L.E.), develops and reformulates physical battery models, and works on methods to simulate and solve these models with greater efficiency and accuracy. By providing a more effective, versatile and accurate model for Li-ion battery technology, M.A.P.L.E. lab’s research can help design batteries more precisely, for safer and more efficient operation.
Related News
- Comparing Lithium-Ion and Sodium-Ion Batteries
- High-Performance 12V Battery Packs | Reliable Energy Solutions
- Home Energy Storage Systems in 2025 Trends, Benefits, and Market Outlook
- The Five Fundamental Design Principles for Energy Storage Cabinets
- Safety Guide for Homes with Solar Power Generation, BESS and EVs
- How Long Do Electric Vehicle Batteries Last? Comparing Lead-Acid & Lithium
- Application of lithium battery in ship
- How to transport lithium batteries more safely?
- New Trends in the Home Energy Storage Market
- This article will show you the difference between prismatic and cylindrical batteries







